Fay Guitars
Simon Fay Guitars
HOW TO CALIBRATE A HYGROMETER
One of the most important tools in a luthier's workshop is an accurate hygrometer. The industry standard of 43% relative humidity is utilized because it allows the instrument to work well in both dry and wet climates. Guitars built to this standard are able to function in the 35 to 55% humidity range without issue - a range achievable in most environments with minor humidification or dehumidification. Move outside this range and you will begin to have playability and/or structural issues. For guitar players, as long as you have a hygrometer that is within 5% of the true reading then you should be just fine.
There are several methods one can use to calibrate a hygrometer but the method I use is a salt-test. I find it to be the easiest and most reliable calibration method. The salt I use is laboratory grade Potassium Carbonate and when used to make a saturated aqueous (water) solution, the relative humidity above the solution will always be 43%. The test is very simple but I have seen some improper instructions on how to prepare the saturated solution. I've made the following pictorial to show exactly how to perform this test and ensure you get accurate results.
**Please note that any reference to salt in the instructions below refers to Potassium Carbonate and not regular table salt (NaCl).
PROCEDURE
1) Get a sealable plastic container.
2) Make a slurry from Potassium Carbonate and water that completely covers the bottom of the container.
3) Place a small plastic platform in the slurry and place the hygrometer on top of it.
4) Close the container and wait 24 hours for the environment inside the container to reach equilibrium.
Step One
I like to use a Tupperware container that forms a good seal. My hygrometer is thin and so I don't need a tall container.
Step Two
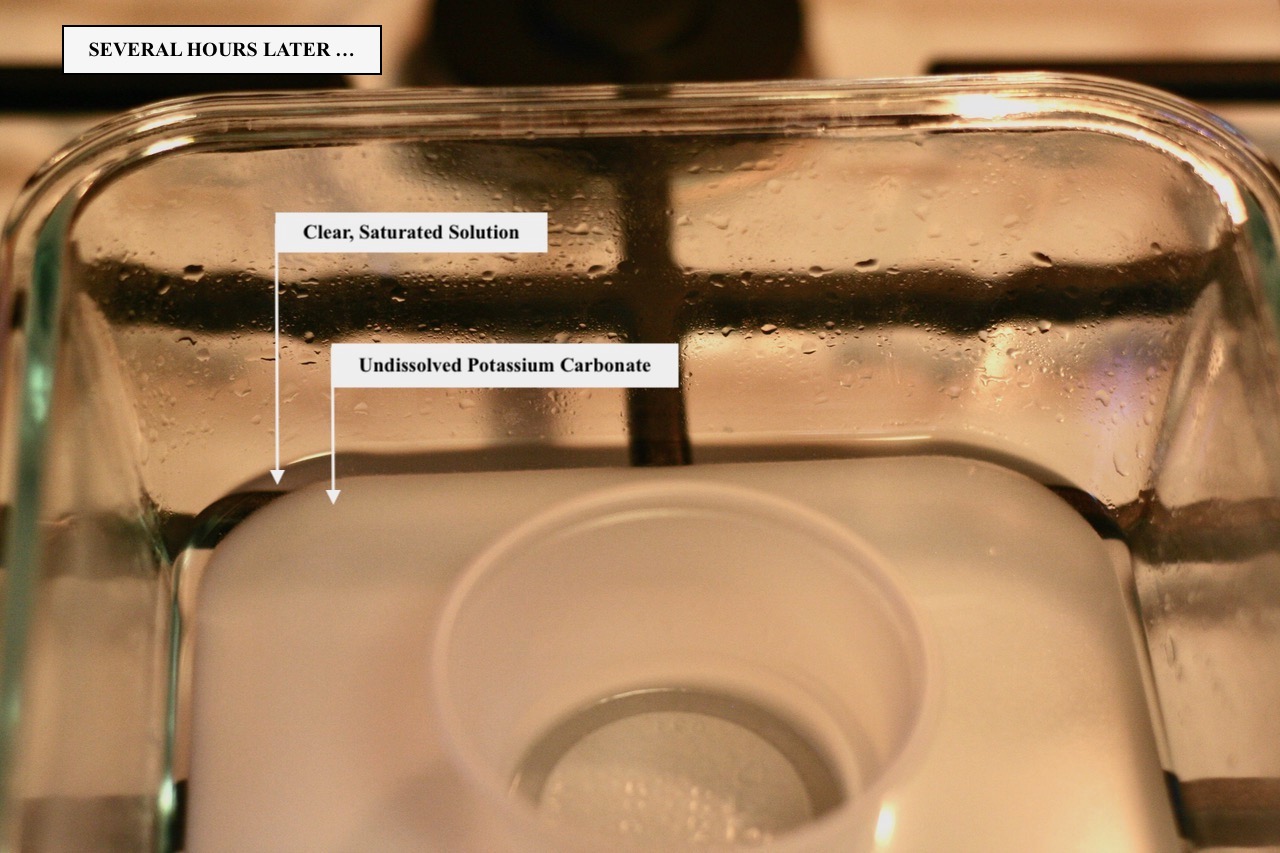
It is important that you create a slurry — a situation where you see both liquid and salt together. If you stir the liquid and see a lot of salt particles suspended in the solution, then you've created a slurry. I've read instructions online that call for dampening the salt with a bit of water but this is not a failsafe approach as it is possible to not add enough water to correctly perform the test. Start by filling the bottom of the container with a 1/4" thick layer of distilled or purified water. Add Potassium Carbonate salt and stop when the water becomes milky/cloudy. Stir the solution thoroughly for a minute or two. You should be able to feel any undissolved salt with a spoon as you are stirring. If all the salt dissolves, add more Potassium Carbonate and stir thoroughly. When it is evident no more salt will dissolve, add another 1-2 tablespoons of salt to the solution. You should have some liquid present but a lot of salt visible as well. The solution will remain milky for several hours as there will be a lot of fine particles suspended in the liquid. Several hours later, the undissolved salt will settle and the solution will become clear with a layer of undissolved salt in the bottom of the container.
Step Three & Four
You need a platform so you can place the hygrometer above the salt solution. A small plastic mixing cup is ideally suited for this task. Carefully place the hygrometer in the container and close the lid. The reading tends to spike during the first hour of the test but will quickly reach equilibrium. The test could probably be completed in a few hours but I like to wait overnight and check the reading periodically to ensure it remains stable.
———————
The test is now complete and you can record how far off your hygrometer is from 43% RH. Some hygrometers will allow you to input or adjust the RH display value; however, if your hygrometer doesn't allow for this then you can simply make a notation somewhere of how much to add or subtract to reach the true reading.




© 2023 Simon Fay Guitars